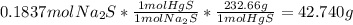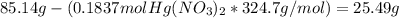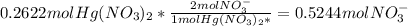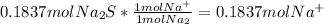Fa solution containing 85.14 g of mercury(ii) nitrate is allowed to react completely with a solution containing 14.334 g of sodium sulfide, how many grams of solid precipitate will be formed? mass: 29.69 g how many grams of the reactant in excess will remain after the reaction? mass: g assuming complete precipitation, how many moles of each ion remain in solution? if an ion is no longer in solution, enter a zero (0) for the number of moles. hg2+ : 0 mol no–3 : 0 mol na+ : 0 mol s2− : 0 mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2


Do you know the correct answer?
Fa solution containing 85.14 g of mercury(ii) nitrate is allowed to react completely with a solution...
Questions in other subjects:






Social Studies, 26.03.2020 04:16





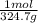 =0.2622 moles.Moles of sodium sulfide = 14.334 g *
=0.2622 moles.Moles of sodium sulfide = 14.334 g *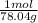 =0.1837 moles.
=0.1837 moles.