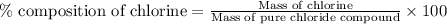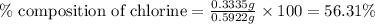Chemistry, 18.09.2019 03:30, shortcake8047
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as agcl by the addition of an excess of silver nitrate. the mass of the resulting agcl is found to be 1.3487 g. what is the mass percentage of chlorine in the original compound?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1


Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride...
Questions in other subjects:


Social Studies, 07.02.2022 15:30



History, 07.02.2022 15:30

Mathematics, 07.02.2022 15:30


Mathematics, 07.02.2022 15:30


History, 07.02.2022 15:30