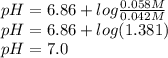Chemistry, 18.09.2019 03:00, tnbankspines
The phosphate buffer system is very important for maintaining the ph of the cytoplasm of all cells. phosphoric acid is a triprotic acid; however, the relevant equilibrium in the biologically useful, neutral range, with a pka of 6.86, is that of dihydrogen phosphate and monohydrogen phosphate ions: h3po4 ⇌ h2po4- + h+ pka = 2.14 h2po4- ⇌ hpo42- + h+ pka = 6.86 hpo42- ⇌ po43- + h+ pka = 12.4 using the henderson-hasselbalch equation, calculate the ph of a solution containing 0.042 m nah2po4 and 0.058 m na2hpo4.

Answers: 2
Other questions on the subject: Chemistry




Do you know the correct answer?
The phosphate buffer system is very important for maintaining the ph of the cytoplasm of all cells....
Questions in other subjects:


Mathematics, 12.03.2021 01:20


Mathematics, 12.03.2021 01:20


Mathematics, 12.03.2021 01:20





![pH=pka+log\frac{[A^{-} ]}{[HA]}](/tpl/images/0238/4057/fae8c.png)
![pH=pka+log\frac{[HPO4^{-2} ]}{[H2PO4^{-} ]}](/tpl/images/0238/4057/ec35f.png)