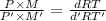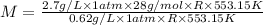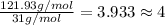Chemistry, 18.09.2019 02:00, davisparker5269
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1 . under the same conditions, nitrogen gas has a density of 0.62 g l–1 . how many atoms of phosphorus are there in one phosphorus molecule under these conditions?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2

Chemistry, 23.06.2019 09:00, littlemoneyh
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
Do you know the correct answer?
At its boiling point (280°c) and at atmospheric pressure, phosphorus gas has a density of 2.7 g l–1...
Questions in other subjects:

Mathematics, 14.01.2020 17:31


Mathematics, 14.01.2020 17:31


Spanish, 14.01.2020 17:31


Biology, 14.01.2020 17:31


History, 14.01.2020 17:31

Mathematics, 14.01.2020 17:31


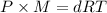 ...(1)
...(1)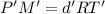
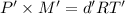 ...(2)
...(2)