
Chemistry, 18.09.2019 01:00, emalvidrez5205
Consider the following reaction: chcl3(g) + cl2(g) → ccl4(g) + hcl(g) the initial rate of the reaction is measured at several different concentrations of the reactants with the following results: [chcl3] (m) [cl2] (m) initial rate (m/s) 0.010 0.010 0.0035 0.020 0.010 0.0069 0.020 0.020 0.0098 0.040 0.040 0.027 from the data, choose the correct rate law for the reaction. rate=k[chcl3][cl2]2 rate=k[chcl3][cl2]12 rate=k[chcl3]2[cl2] rate=k[chcl3]12[cl2]

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
Do you know the correct answer?
Consider the following reaction: chcl3(g) + cl2(g) → ccl4(g) + hcl(g) the initial rate of the react...
Questions in other subjects:


Law, 03.11.2020 01:00






Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00


![\text{Rate}=k[CHCl_3][Cl_2]^{1/2}](/tpl/images/0238/0898/b5249.png)
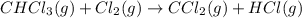
![\text{Rate}=k[CHCl_3]^a[Cl_2]^b](/tpl/images/0238/0898/f7b12.png)
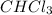
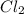
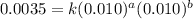 ....(1)
....(1)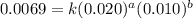 ....(2)
....(2)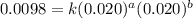 ....(3)
....(3)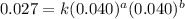 ....(4)
....(4)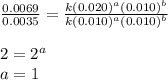
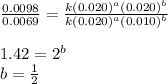
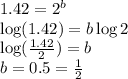
![\text{Rate}=k[CHCl_3]^1[Cl_2]^{1/2}](/tpl/images/0238/0898/0ea6b.png)




