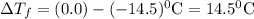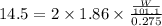Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at −14.5 ∘c? the freezing point for pure water is 0.0 ∘c and kf is equal to 1.86 ∘c/m. express your answer to three significant figures and include the appropriate units.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1



Chemistry, 23.06.2019 05:00, lraesingleton
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
Do you know the correct answer?
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of wate...
Questions in other subjects:

Mathematics, 25.09.2019 01:30

Mathematics, 25.09.2019 01:30


Biology, 25.09.2019 01:30

Mathematics, 25.09.2019 01:30

Social Studies, 25.09.2019 01:30


Mathematics, 25.09.2019 01:30



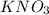 must be added
must be added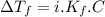
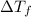 is depression in freezing point of solution, i is van't hoff factor (equal to number of ions produce from dissociation of 1 molecule of electrolyte) and C is molality of solutionMolar mass of
is depression in freezing point of solution, i is van't hoff factor (equal to number of ions produce from dissociation of 1 molecule of electrolyte) and C is molality of solutionMolar mass of 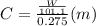 For
For 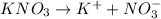 )Here
)Here