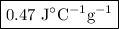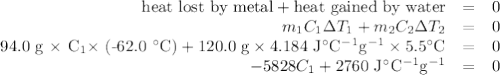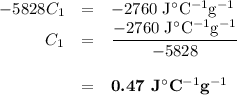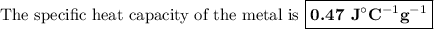A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the final temperature in the calorimeter is 32.0 °c. determine the specific heat of the metal. show your work by listing various steps, and explain how the law of conservation of energy applies to this situation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1


Chemistry, 23.06.2019 15:30, expeditionofsin
How many moles of potassium nitrate, kno3 are present in a sample with a mass of 85.2 g?
Answers: 1
Do you know the correct answer?
A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the...
Questions in other subjects:


Mathematics, 30.06.2019 10:50

Geography, 30.06.2019 10:50



Social Studies, 30.06.2019 10:50