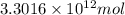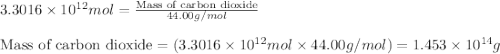Chemistry, 17.09.2019 20:10, samanthasheets8006
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (in grams) of co2 produced by the combustion of 1.80 x 1010 gallons of gasoline (the estimated annual consumption of gasoline in the u. remember, there are 3.785 liters in 1 gallon and assume that isooctane is the only carbon containing component of gasoline. scientific notation can be entered as follows: 1.23 x 1023 = 1.23e23

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Do you know the correct answer?
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (i...
Questions in other subjects:

History, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

History, 27.12.2019 16:31


History, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

Biology, 27.12.2019 16:31

English, 27.12.2019 16:31

Mathematics, 27.12.2019 16:31

History, 27.12.2019 16:31


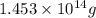
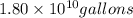
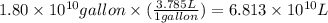
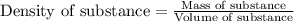
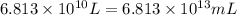 (Conversion factor: 1 L = 1000 mL)
(Conversion factor: 1 L = 1000 mL)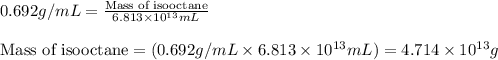
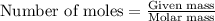 .....(1)
.....(1)
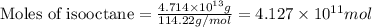
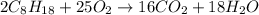
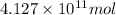 of isooctane will produce =
of isooctane will produce = 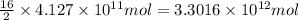 of carbon dioxide
of carbon dioxide