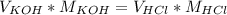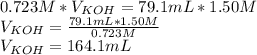Chemistry, 17.09.2019 19:00, chaseking120418
Titration is a type of experiment that can be performed to investigate a neutralization reaction. the equivalence point is when all of the acid and base is fully neutralized. a sample of 0.723 m aqueous potassium hydroxide was titrated against a standard solution of hydrochloric acid. what was the volume of the potassium hydroxide solution if 79.1 ml of 1.50 m hydrochloric acid was needed to reach the equivalence point?

Answers: 3
Similar questions

Chemistry, 24.07.2019 14:00, enrique3300
Answers: 1

Mathematics, 11.09.2019 01:30, PrinceBaphomet
Answers: 2

Chemistry, 10.10.2019 06:20, zel990252
Answers: 1

Chemistry, 24.10.2019 03:50, ziar7176
Answers: 1
Do you know the correct answer?
Titration is a type of experiment that can be performed to investigate a neutralization reaction. th...
Questions in other subjects:

English, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01






Mathematics, 21.10.2020 01:01