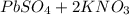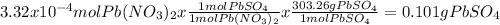Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
Do you know the correct answer?
When aqueous solutions of k₂so₄ and pb(no₃)₂ are combined, pbso₄ precipitates. calculate the mass, i...
Questions in other subjects:


History, 03.07.2019 23:10

History, 03.07.2019 23:10

Mathematics, 03.07.2019 23:10

Mathematics, 03.07.2019 23:10

History, 03.07.2019 23:10


Biology, 03.07.2019 23:10

History, 03.07.2019 23:10


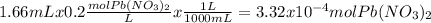
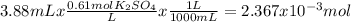
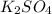
 ⇒
⇒