
Chemistry, 16.09.2019 17:10, briweaver9993
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2co the reaction of 7.39 kg titanium dioxide with excess c and cl₂ gives 14.24 kg titanium tetrachloride. calculate the theoretical yield of ticl₄ (assuming complete reaction) and its percentage yield.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
Do you know the correct answer?
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2...
Questions in other subjects:



Mathematics, 11.03.2021 14:00

World Languages, 11.03.2021 14:00

History, 11.03.2021 14:00

Physics, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00


Biology, 11.03.2021 14:00


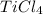 and its percentage yield is 81.0%
and its percentage yield is 81.0%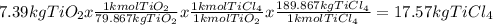
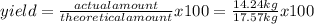 =81.0%
=81.0%




