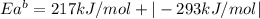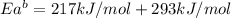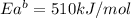Chemistry, 14.09.2019 11:10, joelpimentel
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and the change in enthalpy for the reaction is δh = -293 kj/mol .
what is the activation energy for the reverse reaction?
enter your answer numerically and in terms of kj/mol.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
Do you know the correct answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and th...
Questions in other subjects:

English, 16.12.2020 22:50







Mathematics, 16.12.2020 22:50



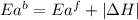
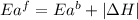
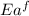 = activation energy for forward reaction
= activation energy for forward reaction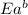 = activation energy for backward reaction
= activation energy for backward reaction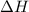 = change in enthalpy of reaction
= change in enthalpy of reaction