

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, creepycrepes
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
Do you know the correct answer?
Data: a h f values: ch 4( g), -74.8 kj; co 21 g), -393.5 kj; h 20( 1), -285.8 kj. using the a h...
Questions in other subjects:


Social Studies, 24.04.2020 03:05

History, 24.04.2020 03:05

Mathematics, 24.04.2020 03:05


History, 24.04.2020 03:05





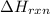 for the given reaction is -890.3 kJ
for the given reaction is -890.3 kJ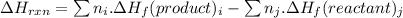
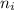 and
and 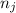 represents number of moles of i-th product and j-th reactant in balanced reaction respectively.
represents number of moles of i-th product and j-th reactant in balanced reaction respectively.![\Delta H_{rxn}=[1mol\times \Delta H_{f}(CO_{2})_{g}]+[2mol\times \Delta H_{f}(H_{2}O)_{l}]-[1mol\times \Delta H_{f}(CH_{4})_{g}]-[2mol\times \Delta H_{f}(O_{2})_{g}]](/tpl/images/0231/1949/6e9b2.png)
![\Delta H_{rxn}=[1mol\times -393.5kJ/mol]+[2mol\times -285.8kJ/mol]-[1mol\times -74.8kJ/mol]+[2mol\times 0kJ/mol]=-890.3 kJ](/tpl/images/0231/1949/b5c4b.png)




