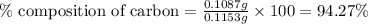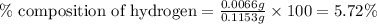Chemistry, 14.09.2019 09:30, bagofmud8339
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gram of co2and 0.0578 gram of h2o. determine the masses of c and h in the sample and the percentages of these elements in this hydrocarbon.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, cristinaledford3696
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1


Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
Do you know the correct answer?
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gra...
Questions in other subjects:

Mathematics, 18.09.2021 03:40

History, 18.09.2021 03:40



Mathematics, 18.09.2021 03:50


Mathematics, 18.09.2021 03:50

Biology, 18.09.2021 03:50




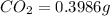

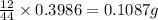 of carbon will be contained.
of carbon will be contained. of hydrogen will be contained.
of hydrogen will be contained. ......(1)
......(1)