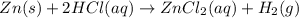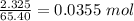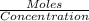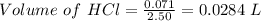Chemistry, 14.09.2019 09:20, miriamnelson7545
Zinc reacts with hydrochloric acid according to the reaction equation shown. zn(s) + 2 hcl(aq) → zncl2(aq) + h2(g) how many milliliters of 2.50 m hcl(aq) are required to react with 4.65 g of an ore containing 50.0% zn(s) by mass? volume: ml

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kristineford198
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1
Do you know the correct answer?
Zinc reacts with hydrochloric acid according to the reaction equation shown. zn(s) + 2 hcl(aq) → znc...
Questions in other subjects:

Mathematics, 04.01.2021 19:10




Mathematics, 04.01.2021 19:10




Social Studies, 04.01.2021 19:10

Social Studies, 04.01.2021 19:10