
Chemistry, 14.09.2019 08:30, postorivofarms
Methods: part a: preparation of buffers make two buffers starting with solid material, which is the most common way to make buffers. you will be given a desired ph, and your task is to prepare 100 ml of two appropriate buffers at a concentration of 0.10 m. one of the buffers will be a phosphate buffer (ph 7.0) and the other will be a tris buffer (ph 8.0). 1. calculate the weight of the buffer you would need to make 100 ml of a 0.10 m solution. weigh out the correct amount and dissolve in 50 ml water. you will need the following compound molecular weights: na2hpo4 (141.96 g/mol), nah2po4 (119.96 g/mol), and tris base (121.1 g/mol).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Do you know the correct answer?
Methods: part a: preparation of buffers make two buffers starting with solid material, which is th...
Questions in other subjects:

Social Studies, 06.12.2019 16:31


Biology, 06.12.2019 16:31


History, 06.12.2019 16:31


Mathematics, 06.12.2019 16:31


Biology, 06.12.2019 16:31

Mathematics, 06.12.2019 16:31


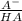
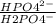
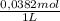 ×
× 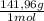 = 0,542 g of Na₂HPO₄
= 0,542 g of Na₂HPO₄ 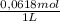 ×
× 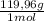 = 0,741 g of NaH₂PO₄
= 0,741 g of NaH₂PO₄ 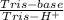
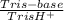 (3)
(3)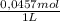 ×
× 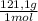 = 0,553 g of Tris-base
= 0,553 g of Tris-base 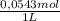 ×
× 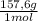 = 0,856 g of Tris-HCl
= 0,856 g of Tris-HCl



