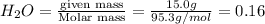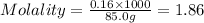Chemistry, 14.09.2019 08:30, trillralyn4060
Calculate the molality of a 150% by mass solution of mgcl, fw-95.3 g/mol in h. o. the density of tis solution is 1.127 gim 0.0134 m 0.157 m 1.58 m 1.86 m 11.8 m igator delete backspace u 10 pilli

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, blondieb1722
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 05:50, starfox5454
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 06:00, bvbbridesmaid5519
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
Do you know the correct answer?
Calculate the molality of a 150% by mass solution of mgcl, fw-95.3 g/mol in h. o. the density of tis...
Questions in other subjects:



Social Studies, 25.09.2019 15:00


Biology, 25.09.2019 15:00

Health, 25.09.2019 15:00

English, 25.09.2019 15:00




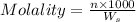
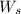 = weight of solvent in g
= weight of solvent in g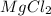 is present in 100 g of solution
is present in 100 g of solution