
Chemistry, 14.09.2019 08:20, serenityarts123
Calculate δs°for the combustion of ammonia.
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substance nh3(g) o2(g) n2(g) h2o(l)
s°(j/k·mol) 192 205.1 192 70
-135 j
-579 j
-387 j
579 j

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1


Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
Calculate δs°for the combustion of ammonia.
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substanc...
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substanc...
Questions in other subjects:



Social Studies, 12.12.2019 23:31





Mathematics, 12.12.2019 23:31



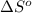 of the reaction is
of the reaction is 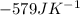
![\Delta S_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0231/1100/ec939.png)
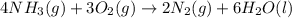
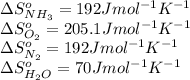
![\Delta S^o_{rxn}=[(6\times \Delta S^o_{H_2O})+(2\times \Delta S^o_{N_2})]-[(4\times \Delta S^o_{NH_3})+(3\times \Delta S^o_{O_2})]](/tpl/images/0231/1100/480ae.png)
![\Delta S^o_{rxn}=[(6\times 70)+(2\times 192)]-[(4\times 192)+(3\times 205.1)]=-579JK^{-1}](/tpl/images/0231/1100/8f98e.png)




