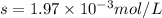Calcium sulfate is only sparingly soluble. caso4(s) ⇌ ca2+(aq) + so42-(aq) for this type of dissolution reaction the equilibrium constant, also known as the solubility product, is denoted ks. in the reaction above, ks = 3.9 x 10-6. when an excess of the solid is dissolved in water what is the maximum concentration of ca2+(aq) in mol l-1?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2


Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
Do you know the correct answer?
Calcium sulfate is only sparingly soluble. caso4(s) ⇌ ca2+(aq) + so42-(aq) for this type of dissolut...
Questions in other subjects:


History, 01.07.2019 02:30


Chemistry, 01.07.2019 02:30





Chemistry, 01.07.2019 02:30

Mathematics, 01.07.2019 02:30


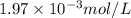
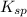
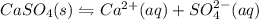
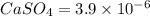
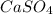 gives 1 mole of
gives 1 mole of 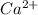 and 1 mole of
and 1 mole of 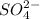 .
.![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0231/0233/958f2.png)
![3.9\times 10^{-6}=[s][s]](/tpl/images/0231/0233/47679.png)