
Chemistry, 14.09.2019 07:10, Ididntwanttomakethis
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) what is the ph before any naoh is added? b) what is the ph after 5.00 ml of naoh are added? c) after 10 ml of naoh are added? d) what is the ph when 20 ml of naoh have been added? what is this point in the titration called?

Answers: 2
Similar questions

Chemistry, 01.07.2019 21:40, isaactatep808nj
Answers: 2

Chemistry, 13.07.2019 00:30, josebrown521
Answers: 1
Do you know the correct answer?
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) wha...
Questions in other subjects:



Mathematics, 30.04.2021 20:20

Mathematics, 30.04.2021 20:20

Social Studies, 30.04.2021 20:20

Chemistry, 30.04.2021 20:20

History, 30.04.2021 20:20

Mathematics, 30.04.2021 20:20

English, 30.04.2021 20:20

English, 30.04.2021 20:20


![\frac{[x][x] }{[0,200-x]}](/tpl/images/0231/0228/4f77c.png)
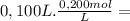 = 2,0x10⁻² mol
= 2,0x10⁻² mol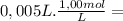 = 5,0x10⁻³ mol
= 5,0x10⁻³ mol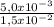
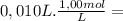 = 1,0x10⁻² mol
= 1,0x10⁻² mol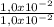
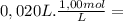 = 2,0x10⁻² mol
= 2,0x10⁻² mol![\frac{[x][x] }{[0,01667-x]}](/tpl/images/0231/0228/2af99.png)




