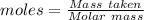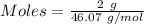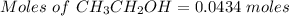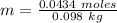Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
Do you know the correct answer?
You are asked to prepare a solution that is 2% by weight ethanol in water. note that the molecular w...
Questions in other subjects:

History, 25.02.2021 23:10


English, 25.02.2021 23:10

Arts, 25.02.2021 23:10



Mathematics, 25.02.2021 23:10


Mathematics, 25.02.2021 23:10


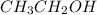 = 2 g
= 2 g