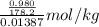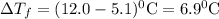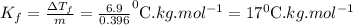A
pure solvent freezes at 12.0 c. a solution of 0.980 g of the solute
and 13.870 g of so...


Answers: 2
Similar questions

Chemistry, 16.10.2019 17:20, gladysvergara
Answers: 2

Chemistry, 16.10.2019 19:00, annie1799
Answers: 2

Chemistry, 16.10.2019 19:00, memphissmith5779
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Mathematics, 08.10.2019 05:30


Biology, 08.10.2019 05:30

Mathematics, 08.10.2019 05:30

Mathematics, 08.10.2019 05:30




Mathematics, 08.10.2019 05:30

Spanish, 08.10.2019 05:30


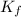 for solvent is
for solvent is 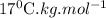
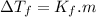 , where
, where  is depression in freezing point and m is molality of solution
is depression in freezing point and m is molality of solution