
Chemistry, 25.12.2019 20:31, tommylopez22713
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(g)
all but one of the conditions will cause equilibrium to shift right and produce more of the products. which change will cause the reaction to shift to the left?
a) adding oxygen
b) adding co2
c) increasing the pressure
d) decreasing the temperature

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
Do you know the correct answer?
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(...
Questions in other subjects:

Mathematics, 27.07.2021 23:30

Mathematics, 27.07.2021 23:30


Spanish, 27.07.2021 23:30







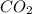
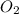 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 



