
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is burned in pure oxygen, an reaction that proceeds according to the following unbalanced equation.
fex(co)y + o2 --> fe2o3 + co2
if you burn 1.959 g. of fex(co)y and obtain 0.799 g. of fe2o3 and 2.200 g. of co2, what is the empirical formula of fex(co)y?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
Do you know the correct answer?
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is bu...
Questions in other subjects:

Biology, 29.06.2019 10:30


Mathematics, 29.06.2019 10:30


Physics, 29.06.2019 10:30

English, 29.06.2019 10:30


Mathematics, 29.06.2019 10:30



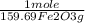 = 5,00×10⁻³ Fe₂O₃ moles
= 5,00×10⁻³ Fe₂O₃ moles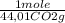 = 5,00×10⁻²CO₂ moles
= 5,00×10⁻²CO₂ moles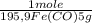 = 1,00×10⁻² Fe(CO)₅ moles
= 1,00×10⁻² Fe(CO)₅ moles



