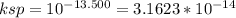Chemistry, 13.09.2019 22:30, praptibaral70
Calculate the number of mg of mn2+ left
unprecipitated in 100 ml of a 0.1000m solution of mnso4
to whichenough na2s has been added to makethe final
sulfide ion (s2-)concentration equal to 0.0900 m. assume
no change in volume due tothe addition of na2s.
thepksp of mns is 13.500.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, nadinealonzo6121
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
Do you know the correct answer?
Calculate the number of mg of mn2+ left
unprecipitated in 100 ml of a 0.1000m solution of mnso...
unprecipitated in 100 ml of a 0.1000m solution of mnso...
Questions in other subjects:


Mathematics, 06.05.2020 16:57

Mathematics, 06.05.2020 16:57

Mathematics, 06.05.2020 16:57


Biology, 06.05.2020 16:57