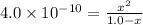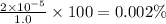Chemistry, 13.09.2019 22:30, natimike10
Hydrocyanic acid has a ka of 4.0
x10-10. what is the percent ofionization of
a 1.0 molar solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, mannster03
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 23.06.2019 09:00, sammypaige08
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2

Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
Do you know the correct answer?
Hydrocyanic acid has a ka of 4.0
x10-10. what is the percent ofionization of
a 1.0 molar...
x10-10. what is the percent ofionization of
a 1.0 molar...
Questions in other subjects:

Chemistry, 04.04.2022 14:50




Mathematics, 04.04.2022 15:20

Biology, 04.04.2022 15:30


History, 04.04.2022 15:50


French, 04.04.2022 16:00


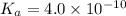
![K_{a}=\frac {\left [ H^{+} \right ]\left [ {CN}^- \right ]}{[HCN]}](/tpl/images/0230/3659/7bd5f.png)