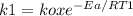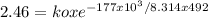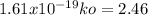Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
Do you know the correct answer?
The specific reaction rate of a reaction at 492k is 2.46 second inverse and at 528k 47.5 second inve...
Questions in other subjects:


Medicine, 12.02.2021 08:10

Biology, 12.02.2021 08:10


Mathematics, 12.02.2021 08:10

Biology, 12.02.2021 08:10

History, 12.02.2021 08:10

Mathematics, 12.02.2021 08:10

Mathematics, 12.02.2021 08:10

Biology, 12.02.2021 08:10


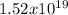 J/mol
J/mol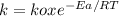
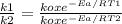
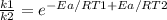
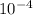
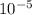 xEa = -2.95
xEa = -2.95