Chemistry, 13.09.2019 05:30, jdodger910
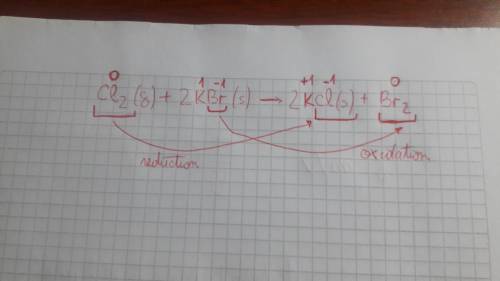
Cl2(g) + 2kbr(s) > 2kcl(s) + br2(g) rewrite the equation and write the color of each chemical under its name. 2. what type of the reaction is that?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2



Chemistry, 23.06.2019 10:30, EstherAbuwaah
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh. the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
Do you know the correct answer?
Cl2(g) + 2kbr(s) > 2kcl(s) + br2(g) rewrite the equation and write the color of each chemical un...
Questions in other subjects:


Mathematics, 25.08.2019 17:30


Health, 25.08.2019 17:30


French, 25.08.2019 17:30


English, 25.08.2019 17:30


Mathematics, 25.08.2019 17:30