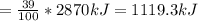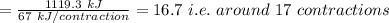Assume that the complete combustion of one mole of glucose to carbon dioxide and water liberates 2870 kj/mol2870 kj/mol ( δ°′=−2870 kj/molδg°′=−2870 kj/mol ). if one contraction cycle in muscle requires 67 kj67 kj , and the energy from the combustion of glucose is converted with an efficiency of 39%39% to contraction, how many contraction cycles could theoretically be fueled by the complete combustion of one mole of glucose? round your answer to the nearest whole number.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 05:00, kayranicole1
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
Do you know the correct answer?
Assume that the complete combustion of one mole of glucose to carbon dioxide and water liberates 287...
Questions in other subjects:



Chemistry, 11.12.2020 01:00

History, 11.12.2020 01:00


Chemistry, 11.12.2020 01:00


History, 11.12.2020 01:00