
Chemistry, 12.09.2019 21:30, abbygutierrez401
For a reaction a + b → products, the following data were collected. experiment number initial concentration of a (m) initial concentration of b (m) observed initial rate (m/s) 1 3.40 4.16 1.82 ✕ 10−4 2 4.59 4.16 3.32 ✕ 10−4 3 3.40 5.46 1.82 ✕ 10−4 calculate the rate constant for this reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
Do you know the correct answer?
For a reaction a + b → products, the following data were collected. experiment number initial concen...
Questions in other subjects:

Social Studies, 22.04.2021 23:40


Mathematics, 22.04.2021 23:50


Chemistry, 22.04.2021 23:50


Computers and Technology, 22.04.2021 23:50

Spanish, 22.04.2021 23:50

Mathematics, 22.04.2021 23:50


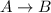
![Rate = k[A]^{x}[B]^{y}](/tpl/images/0229/1912/fc4ee.png)
![\frac{3.32*10^{-4} }{1.82*10^{-4} } =\frac{[4.59]^{x} [4.16]^{y} }{[3.40]^{x} [4.16]^{y} }\\\\x =2](/tpl/images/0229/1912/13d15.png)
![\frac{1.82*10^{-4} }{1.82*10^{-4} } =\frac{[3.40]^{x} [5.46]^{y} }{[3.40]^{x} [4.16]^{y} }\\\\y =0](/tpl/images/0229/1912/8a803.png)
![Rate = k[A]^{2}[B]^{0}](/tpl/images/0229/1912/31aa9.png)
![k = \frac{Rate1}{[A]^{2} } =\frac{1.82*10^{-4}M/s }{[3.40]^{2} } =1.57*10^{-5} s-1](/tpl/images/0229/1912/557a1.png)





