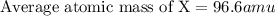An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass of 95.0 amu, 16.90% of isotope x-96 with a mass of 96.0 amu, and 72.42% of isotope x-97 with a mass of 97.0 amu. calculate the atomic mass (in amu) of the element. type in your answer with 3 significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 02:30, paulinahunl17
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
Do you know the correct answer?
An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass o...
Questions in other subjects:


Mathematics, 10.04.2020 16:51

Mathematics, 10.04.2020 16:51



Chemistry, 10.04.2020 16:51



Arts, 10.04.2020 16:51


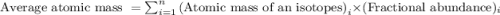 .....(1)
.....(1)![\text{Average atomic mass of X}=[(95\times 0.1068)+(96\times 0.1690)+(97\times 0.7242)]](/tpl/images/0229/1284/93747.png)