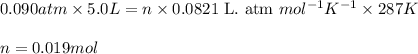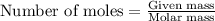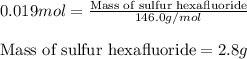Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.090atm . calculate the mass and number of moles of sulfur hexafluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Averybeam300
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3
Do you know the correct answer?
Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l....
Questions in other subjects:


Chemistry, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00

Chemistry, 10.11.2020 14:00


Mathematics, 10.11.2020 14:00

English, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00


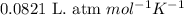
![14^oC=[273+14]K=287K](/tpl/images/0229/0798/930e3.png)