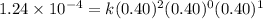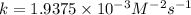Chemistry, 12.09.2019 20:10, ayoismeisjjjjuan
For the reaction a+b+c=> d+e, the initial reaction rate was measured for various initial concentrations of reactants. the following data were collected:
trial a(m) ( c(m) initial rate(m/s)
1 0.40 0.40 0.40 1.2 x 10^-4
2 .40 0.40 .20 .6 x 10^-4
0.80 . 0.40 4.8 x 10^-4
4 0.80 .80 .40 .8 x 10^-4
what is the value of the rate constant k for this reaction?

Answers: 3
Similar questions

Chemistry, 10.09.2019 18:20, aekent2003
Answers: 2

Chemistry, 01.10.2019 04:30, britneywells14ozzp3r
Answers: 2

Chemistry, 02.11.2019 03:31, aboatright7410
Answers: 3
Do you know the correct answer?
For the reaction a+b+c=> d+e, the initial reaction rate was measured for various initial concentr...
Questions in other subjects:

Mathematics, 30.10.2020 20:50


Mathematics, 30.10.2020 20:50

English, 30.10.2020 20:50




Spanish, 30.10.2020 20:50

Health, 30.10.2020 20:50

Geography, 30.10.2020 20:50


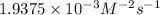
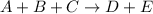
![\text{Rate}=k[A]^a[B]^b[C]^c](/tpl/images/0229/0682/be89a.png)
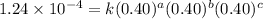 ....(1)
....(1)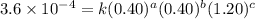 ....(2)
....(2)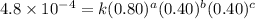 ....(3)
....(3)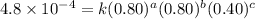 ....(4)
....(4)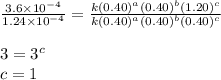
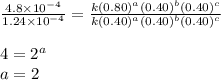
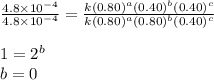
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0229/0682/54afd.png)