
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, cr50, cr52, cr53, and cr54. the cr52 isotope has a natural abundance of 83.79% and an atomic mass of 51.9405 u. the cr54 isotope has a natural abundance of 2.37% and an atomic mass of 53.9389 u. the natural abundances of the cr50 and cr53 isotopes exist in a ratio of 1: 0.4579, and the cr50 isotope has an atomic mass of 49.9460 u. determine the atomic mass of the cr53 isotope.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
Do you know the correct answer?
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, cr50, cr52, cr53, and cr54....
Questions in other subjects:





Mathematics, 08.07.2019 02:00


Mathematics, 08.07.2019 02:00




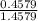 x 13.84 = 4.3469%
x 13.84 = 4.3469%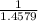 x 13.84u = 9.4931%
x 13.84u = 9.4931%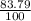 ) + ( 49.9460 x
) + ( 49.9460 x 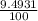 + (m₅₃ x
+ (m₅₃ x 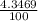 ) + (53.9389 x
) + (53.9389 x 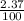 )
)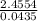 = 56.4459u
= 56.4459u



