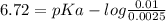Chemistry, 12.09.2019 05:30, amakayla57
An unknown compound, x is thought to have a carboxyl group with a pka of 2.0 and another ionizable group with a pka between 5 and 8. when 75 ml of 0.1 m naoh was added to 100ml of a 0.1 m solution of x at ph 2.0, the ph increased to 6.72. calculate the pka of the second group of x.
source

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Do you know the correct answer?
An unknown compound, x is thought to have a carboxyl group with a pka of 2.0 and another ionizable g...
Questions in other subjects:

Mathematics, 09.12.2020 22:30

English, 09.12.2020 22:30


History, 09.12.2020 22:30

Mathematics, 09.12.2020 22:30

Chemistry, 09.12.2020 22:30

Mathematics, 09.12.2020 22:30

History, 09.12.2020 22:30

Biology, 09.12.2020 22:30


![pH = -log[H^{+}]](/tpl/images/0228/7557/8d3ec.png) , and pKa = -logKa. Ka is the equilibrium constant of the acid.
, and pKa = -logKa. Ka is the equilibrium constant of the acid. ![pH = pKa - log \frac{[HA]}{[A^{-}]}](/tpl/images/0228/7557/93c05.png)
![[A^{-}]](/tpl/images/0228/7557/fe74d.png) is the concentration of the anion which forms the acid.
is the concentration of the anion which forms the acid.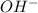 will be
will be