
Chemistry, 12.09.2019 01:10, maehardy4134
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.60g of sodium carbonate is mixed with one containing 5.14 of silver nitrate.
1.) how many grams of sodium carbonate are present after the reaction is complete?
2.)how many grams of silver nitrate are present after the reaction is complete?
3.)how many grams of silver carbonate are present after the reaction is complete?
4.) how many grams of sodium nitrate are present after the reaction is complete?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
Do you know the correct answer?
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution...
Questions in other subjects:


English, 23.10.2020 08:01



English, 23.10.2020 08:01

Mathematics, 23.10.2020 08:01

Mathematics, 23.10.2020 08:01



Mathematics, 23.10.2020 08:01


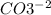 , and the ion nitrate is
, and the ion nitrate is 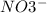 .
. 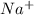 . Silver has only one electron too, so the cation will be
. Silver has only one electron too, so the cation will be  .
. 



