
Chemistry, 11.09.2019 04:30, revlonknox6
The following substances dissolve when added to water. classify the substances according to the strongest solute-solvent interaction that will occur between the given substances and water during dissolution.
(1) ion-ion forces
(2) dipole dipole forces
(3) ion dipole forces
(4) london dispersion forces
(a) hf
(b) ch3oh
(c) cacl2
(d) febr3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
Do you know the correct answer?
The following substances dissolve when added to water. classify the substances according to the stro...
Questions in other subjects:

Mathematics, 21.06.2019 14:30








Mathematics, 21.06.2019 14:30


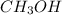 - There will also be development of partial charges due to the difference in electronegativity of oxygen and hydrogen atoms.
- There will also be development of partial charges due to the difference in electronegativity of oxygen and hydrogen atoms. 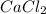 - It is an ionic compound. Hence, there will be partial positive charge on calcium and partial negative charge on chlorine atom.
- It is an ionic compound. Hence, there will be partial positive charge on calcium and partial negative charge on chlorine atom.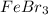 - It is an ionic compound. Hence, there will also exist ion-ion forces.
- It is an ionic compound. Hence, there will also exist ion-ion forces.



