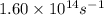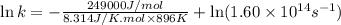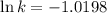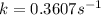Chemistry, 11.09.2019 04:30, shawn20034
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energy is 249 kj/mol and the frequency factor is 1.60 × 1014 s−1. find the value of the specific rate constant at 896 k . enter your answer numerically (to 4 decimal places) and in terms of the appropriate units for a first order reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, PineaPPle663
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1
Do you know the correct answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energ...
Questions in other subjects:

Mathematics, 21.04.2021 23:50



Chemistry, 21.04.2021 23:50


History, 21.04.2021 23:50

English, 21.04.2021 23:50


Business, 21.04.2021 23:50


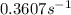
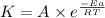
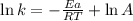 ............(1)
............(1)