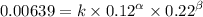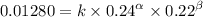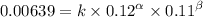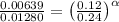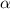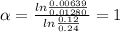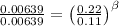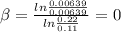Chemistry, 11.09.2019 03:10, taminazaka1
Reaction rates
part a
for the arbitrary reaction,
a + b ? c + d
the following initial rates were measured given the initial concentrations of a and b. determine the rate order for both a and b.
[a]o [b]o initial rate (m/s)
0.12 0.22 0.00639
0.24 0.22 0.0128
0.12 0.11 0.00639
part b
-0th order in a and 1st order in b
-2nd order in a and 0th order in b
-1st order in a and 1st order in b
-1st order in a and 0th order in b
the following arbitrary reaction is exothermic:
a + b ? c + d
predict what will happen to the rate of the reaction if the temperature is increased.
-the reaction rate will decrease.
-equilibrium is shifted to the left.
-the reaction rate increases.
-there will be no change in rate.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
Do you know the correct answer?
Reaction rates
part a
for the arbitrary reaction,
a + b ? c + d
the fol...
part a
for the arbitrary reaction,
a + b ? c + d
the fol...
Questions in other subjects:

Chemistry, 10.12.2020 19:10




Mathematics, 10.12.2020 19:10

Biology, 10.12.2020 19:10

Mathematics, 10.12.2020 19:10

Mathematics, 10.12.2020 19:10



![-r_A=k\times\left[A\right]^\alpha\times\left[B\right]^\beta\bigm](/tpl/images/0227/4892/e6011.png)