
Chemistry, 11.09.2019 03:10, cmflores3245
Asample of pure water is heated to a temperature of 112 c at a pressure of 20 mpa, where the ionization constant for water is 4.0 x 1012. what is the oh concentration in the pure water at these conditions?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1

Chemistry, 23.06.2019 10:10, nancysue1975
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
Do you know the correct answer?
Asample of pure water is heated to a temperature of 112 c at a pressure of 20 mpa, where the ionizat...
Questions in other subjects:



Mathematics, 29.12.2019 03:31

History, 29.12.2019 03:31


Physics, 29.12.2019 03:31


World Languages, 29.12.2019 03:31

Computers and Technology, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31


![[OH^-]^2=2.0\times 10^{6}](/tpl/images/0227/4886/54276.png)
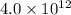 at a temperature of
at a temperature of 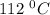 and pressure of 20 MPa
and pressure of 20 MPa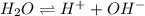
![K_w=[H^+][OH^-]](/tpl/images/0227/4886/bc68a.png)
![[H^+]=[OH^-]](/tpl/images/0227/4886/0669d.png)
![K_w=[OH^-]^2](/tpl/images/0227/4886/d08d4.png)
![[OH^-]^2=4.0\times 10^{12}](/tpl/images/0227/4886/f96fa.png)




