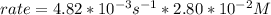Chemistry, 11.09.2019 00:30, nicholasryanencarnac
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at 64 ∘c the rate constant is 4.82 ×10−3s−1. part a part complete write the rate law for the reaction. write the rate law for the reaction. rate=2.41×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5] rate=9.64×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5]2 previous answers correct part b what is the rate of reaction when [n2o5]= 2.80×10−2 m ?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at...
Questions in other subjects:



Arts, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50


Social Studies, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50


English, 29.01.2021 03:50


![rate= 4.82*10^{-3}s^{-1} * [N2O5]](/tpl/images/0227/3013/f16e4.png)
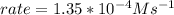
![rate= k * [N2O5]^{x}](/tpl/images/0227/3013/f017a.png)