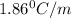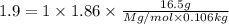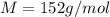Chemistry, 10.09.2019 23:10, blakestuhan
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g of water was found to have a freezing point of -1.9 ∘c. calculate the molar mass of the unknown compound.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1
Do you know the correct answer?
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g o...
Questions in other subjects:





Mathematics, 06.05.2020 20:01


Geography, 06.05.2020 20:01

History, 06.05.2020 20:01

Social Studies, 06.05.2020 20:01


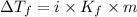
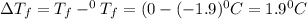 = Depression in freezing point
= Depression in freezing point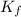 = freezing point constant =
= freezing point constant =