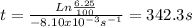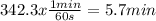Chemistry, 10.09.2019 22:30, liltinyhead
Acertain first-order reaction (a→products) has a rate constant of 8.10×10−3 s−1 at 45 ∘c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration? express your answer with the appropriate units.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
Do you know the correct answer?
Acertain first-order reaction (a→products) has a rate constant of 8.10×10−3 s−1 at 45 ∘c. how many m...
Questions in other subjects:






English, 17.04.2021 15:20


Computers and Technology, 17.04.2021 15:20

Mathematics, 17.04.2021 15:20


![Ln [A] = -k.t + Ln [A]_{0}](/tpl/images/0227/1833/30b90.png) . Where [A] is the concentration of the reactant at any t time of the reaction,
. Where [A] is the concentration of the reactant at any t time of the reaction, ![[A]_{0}](/tpl/images/0227/1833/48818.png) is the concentration of the reactant at the beginning of the reaction and k is the rate constant.
is the concentration of the reactant at the beginning of the reaction and k is the rate constant. ![[A]=\frac{6.25}{100}.[A]_{0}](/tpl/images/0227/1833/4f27b.png) . And the rate constant (k) is 8.10×10−3 s−1
. And the rate constant (k) is 8.10×10−3 s−1![Ln \frac{6.25}{100}.[A]_{0} = -8.10x10^{-3}s^{-1}.t + Ln[A]_{0}](/tpl/images/0227/1833/587cc.png)
![Ln [A]_{0}.\frac{6.25}{100} - Ln [A]_{0} = -8.10x10^{-3}s^{-1}.t](/tpl/images/0227/1833/49854.png)
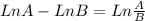
![Ln \frac{[A]_{0}}{[A]_{0}}.\frac{6.25}{100} = -8.10x10^{-3}s^{-1}.t](/tpl/images/0227/1833/72778.png)