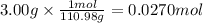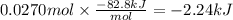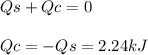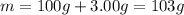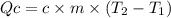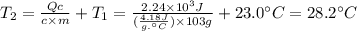Chemistry, 10.09.2019 19:30, santos200154
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial temperature of the calorimeter is 23.0 ∘c. if 3.00 g of cacl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? the heat of solution δhsoln of cacl2 is −82.8 kj/mol. assume that the specific heat of the solution form

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
Do you know the correct answer?
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial...
Questions in other subjects:




Mathematics, 31.07.2019 08:00

Mathematics, 31.07.2019 08:00


English, 31.07.2019 08:00

Mathematics, 31.07.2019 08:00

Health, 31.07.2019 08:00