Chemistry, 10.09.2019 18:30, keirarae2005
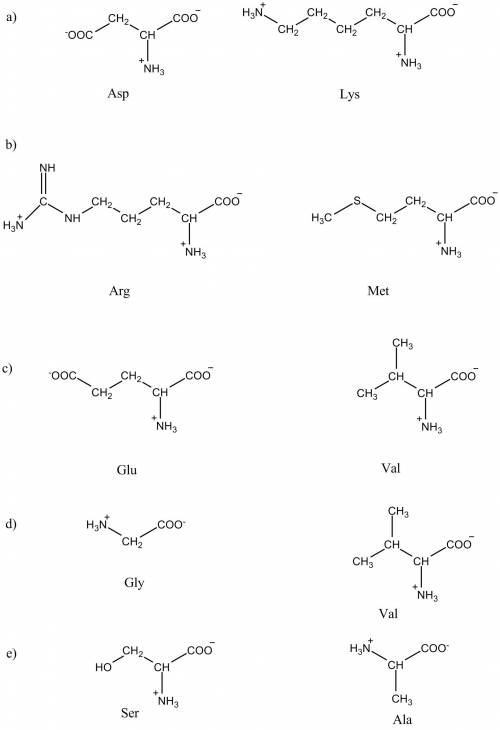
1. separation of amino acids by ion-exchange chromatography. mixtures of amino acids can be analyzed by first separating the mixture into its components through ion-exchange chromatography. amino acids placed on a cation-exchange resin containing sulfate (-so3-) groups flow down the column at different rates because of two factors that influence their movement (1) ionic attraction between the sulfonate residues on the column and positively charged functional groups on the amino acids, and (2) hydrophobic interactions between amino acid side chains and the strongly hydrophobic backbone of the polystyrene resin. for each pair of amino acids listed, determine which will be eluted first from the cation-exchange column by a ph 7.0 buffer.
a.) asp and lys
b.) arg and met
c.) glu and val
d.) gly and val
e.) ser and ala

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 07:30, bryantjorell
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2

Chemistry, 23.06.2019 12:40, ashlpiriz123
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1
Do you know the correct answer?
1. separation of amino acids by ion-exchange chromatography. mixtures of amino acids can be analyzed...
Questions in other subjects:

History, 03.12.2020 22:50



Spanish, 03.12.2020 22:50



Mathematics, 03.12.2020 22:50



Mathematics, 03.12.2020 22:50