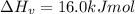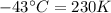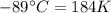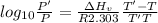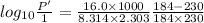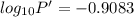Chemistry, 10.09.2019 01:30, natetheman7740
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. calculate the vapor pressure of x at −89.°c. round your answer to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2


Do you know the correct answer?
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. ca...
Questions in other subjects:


History, 22.04.2021 04:30


Physics, 22.04.2021 04:30


Physics, 22.04.2021 04:30

Mathematics, 22.04.2021 04:30

English, 22.04.2021 04:30

Mathematics, 22.04.2021 04:30


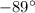 = 0.12 atm
= 0.12 atm