
Chemistry, 09.09.2019 23:30, jennypenny123
Which of the following statements are true concerning ionic bonding? select one: a. ionic bonding occurs between a metal, which has a high affinity for electrons, and a nonmetal, which loses electrons relatively easy. b. cacl2 forms because ca2+ is always a more stable species than the calcium atom alone. c. compounds with ionic bonds tend to have low melting points. d. the electronegativity difference between the bonding atoms of ionic compounds is small since the electrons are not shared but rather held together by electrostatic forces. e. all of the above statements are false.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
Do you know the correct answer?
Which of the following statements are true concerning ionic bonding? select one: a. ionic bonding...
Questions in other subjects:

History, 06.04.2020 22:12


History, 06.04.2020 22:13





English, 06.04.2020 22:13

History, 06.04.2020 22:13


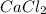 forms because
forms because 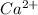 is always a more stable species than the calcium atom alone
is always a more stable species than the calcium atom alone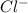 is an anion with oxidation state of -1. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -1. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral ![[Ca]=1s^22s^22p^63s^23p^64s^2](/tpl/images/0226/1839/cdc07.png)
![[Ca^{2+}]=1s^22s^22p^63s^23p^6](/tpl/images/0226/1839/04411.png)
![[Cl]=1s^22s^22p^63s^23p^5](/tpl/images/0226/1839/7a3e4.png)
![[Cl^-]=1s^22s^22p^63s^23p^6](/tpl/images/0226/1839/bea8e.png)





