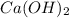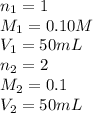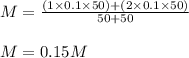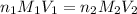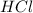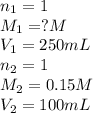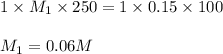Chemistry, 09.09.2019 23:20, snikergrace
We make a basic solution by mixing 50. ml of 0.10 m naoh and 50. ml of 0.10 m ca(oh)2. it requires 250 ml of an hcl solution to neutralize this basic solution. what is the molarity of the hcl solution?
1. 0.015 m2. 0.043 m3. 0.0037 m4. 0.040 m5. 0.060 m

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Do you know the correct answer?
We make a basic solution by mixing 50. ml of 0.10 m naoh and 50. ml of 0.10 m ca(oh)2. it requires 2...
Questions in other subjects:

Mathematics, 26.04.2020 00:28



Spanish, 26.04.2020 00:28




Mathematics, 26.04.2020 00:28

Chemistry, 26.04.2020 00:29

English, 26.04.2020 00:29


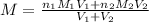
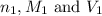 are the n-factor, molarity and volume of the NaOH.
are the n-factor, molarity and volume of the NaOH.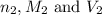 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the