
Chemistry, 09.09.2019 22:30, sarahlearn3
Calculate the osmotic pressure (in torr) of 6.00 l of an aqueous 0.958 m solution at 30.°c, if the solute concerned is totally ionized into three ions (e. g., it could be na2so4 or mgcl2).

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
Calculate the osmotic pressure (in torr) of 6.00 l of an aqueous 0.958 m solution at 30.°c, if the s...
Questions in other subjects:


Spanish, 26.06.2019 03:30


Spanish, 26.06.2019 03:30

Spanish, 26.06.2019 03:30






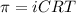
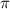 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?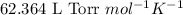
![30^oC=[30+273]K=303K](/tpl/images/0226/1460/fd4b3.png)






