Which of the following solutes will lower the freezing point of water the most?
a.
the...

Chemistry, 09.09.2019 19:30, reaperdraws
Which of the following solutes will lower the freezing point of water the most?
a.
the molecular compound sucrose (c12h22o11)
b.
the ionic compound magnesium sulfate (mgso4)
c.
the ionic compound lithium chloride (licl)
d.
the ionic compound calcium fluoride (caf2)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 16.10.2020 08:01

Spanish, 16.10.2020 08:01

Computers and Technology, 16.10.2020 08:01



Social Studies, 16.10.2020 08:01


Spanish, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01


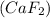
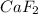 and the freezing point will be lowest.
and the freezing point will be lowest.



